World News
Vaccines for Young Children – The New York Times
-

 General News1 week ago
General News1 week agoEXPOSED: How Kenya’s Student Leaders Have Been Silenced as Lecturers’ Strike Enters Another Week
-
Politics1 week ago
Paul Biya’s Re-Election: What Cameroon’s Political Longevity Reveals About Leadership and Governance in Africa
-

 Politics1 week ago
Politics1 week agoInfluencers for Sale: Inside the Government’s Attempt to Control Public Opinion Through the Cyber Crimes Bill 2025
-

 Business News6 days ago
Business News6 days agoHow Powerful Politicians Are Using Proxies to Privatize Kenya’s Public Assets
-
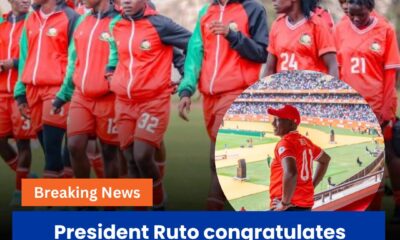
 General News6 days ago
General News6 days agoPresident Ruto Congratulates Harambee Starlets, Pledges Sh1 Million Each After Historic WAFCON Qualification
-

 Business News5 hours ago
Business News5 hours ago15 Proven Ways to Make Money Online in Kenya; No Scams!